Health Topics

The miracle of an artificial pancreas
Four NIH-funded artificial pancreas research efforts are underway
Thanks to investments in research, new and improved methods for managing type 1 diabetes are on the horizon, including the artificial pancreas.
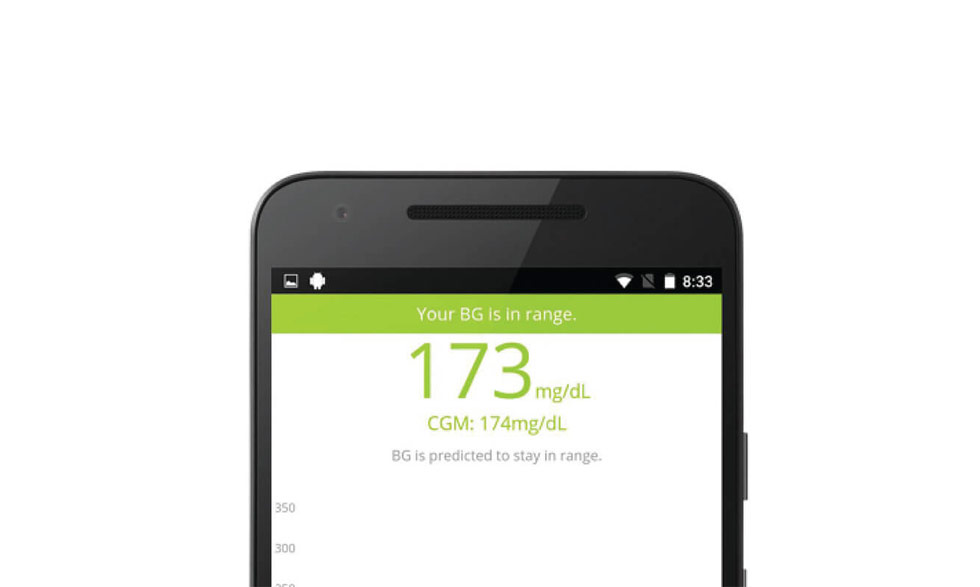
The artificial pancreas is an integrated system that monitors blood glucose (sugar) levels automatically and provides insulin or a combination of insulin and a second hormone to people with type 1 diabetes.
A successful artificial pancreas would be a life-changing advance for many people with type 1 diabetes. This closed-loop system would replace reliance on testing by fingerstick or continuous monitoring systems and separate, non-integrated delivery of insulin by shots or a pump.
The first of several major research efforts to test and refine artificial pancreas systems is now underway. Four separate projects, funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), are designed to be the potential last steps between testing the automated devices and requesting regulatory approval for permanent use.
"These studies aim to collect the data necessary to bring artificial pancreas technology to the people who need it," said Guillermo Arreaza-Rubín, M.D., director of NIDDK's Diabetes Technology Program. "Results from these studies could change and save lives."
Previously, researchers and participants worked together to test artificial pancreas devices in short-term trials, with varying levels of patient supervision. In 2016, the U.S. Food and Drug Administration approved a hybrid model of an artificial pancreas, an automated system that requires users to adjust insulin intake at mealtimes. A fully automated system will sense rising glucose levels, including at mealtimes, and adjust insulin automatically.
In addition to easing the burden of management for people with type 1 diabetes or their caregivers, in shorter studies, the devices brought glucose levels closer to normal than traditional management. NIH research has found that early, tight control of blood glucose helps reduce diabetes complications including nerve, eye, and kidney diseases.
The four research projects begin this year and in 2018. They will be conducted in larger groups over longer periods of time than the earlier trials, and in largely unrestricted conditions. The participants will live at home and monitor themselves, going about their normal lives, with remote monitoring by study staff.
"Managing type 1 diabetes currently requires a constant juggling act between checking blood glucose levels frequently and delivering just the right amount of insulin while taking into account meals, physical activity, and other aspects of daily life, where a missed or wrong delivery could lead to potential complications," said Andrew Bremer, M.D., Ph.D., the NIDDK program official overseeing the studies. "Unifying the management of type 1 diabetes into a single, integrated system could lift so much of that burden."
Studies will look at factors including safety, efficacy, user-friendliness, physical and emotional health of the participants, and cost. The Jaeb Center for Health Research in Tampa, Florida, will serve as coordinating center for all of the trials.
"For many people with type 1 diabetes, the realization of a successful, fully automated artificial pancreas is a dearly held dream. It signifies a life freer from nightly wake-up calls to check blood glucose or deliver insulin, a life freer from dangerous swings of blood glucose," said NIDDK director Griffin P. Rodgers, M.D., M.A.C.P. "Nearly 100 years since the discovery of insulin, a successful artificial pancreas would mark another huge step toward better health for people with type 1 diabetes."
The studies include:
- Now recruiting, the International Diabetes Closed-Loop trial, led by Boris Kovatchev, Ph.D., and Stacey Anderson, M.D., of the University of Virginia, Charlottesville, will test an automated insulin delivery system called inControl. The trial, which uses smartphones, will follow 240 people age 14 and up with type 1 diabetes for six months. A second, six-month study will recruit from the 180 U.S. participants of the first trial to test an alternative algorithm. (NIH grant DK108483)
- This year, recruitment will begin for youths between the ages of six and 18 for a full-year trial of an artificial pancreas. Led by Roman Hovorka, Ph.D., of the University of Cambridge, England, the study seeks to enroll 130 youths for a full year of use of an artificial pancreas system that uses a smartphone as one component. (NIH grant DK108520)
- Starting in late 2017, research on Comparing Two Automated Insulin Delivery System Algorithms in Adolescents and Young Adults With Type 1 Diabetes led by Richard Bergenstal, M.D., of International Diabetes Center, Minneapolis, Minnesota, and Moshe Phillip, M.D., of Schneider Children's Medical Center, Petah Tikva, Israel, will compare the FDA-approved hybrid artificial pancreas to a next-generation system to further improve glucose control, particularly around mealtime. (NIH grant DK108611)
- In mid-2018, a study led by Steven Russell, M.D., Ph.D., of the Massachusetts General Hospital, Boston, and Ed Damiano, M.D., of Boston University will enroll 312 people ages 18 and older. The six-month study will use a bihormonal "bionic pancreas" system, with a dual-chamber pump to deliver both insulin and its counteracting hormone, glucagon, using tested algorithms for automated dual hormone delivery. (NIH grant DK108612) Learn more at the Bionic Pancreas Team website.
The trials are made possible through the Special Statutory Funding Program for Type 1 Diabetes, a congressional appropriation administered by NIDDK to support research to prevent and cure type 1 diabetes and its complications. Together, the grants total about $41 million.