Health Topics
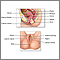
A cone biopsy (conization) is surgery to remove a sample of abnormal tissue from the cervix. The cervix is the lower part of the uterus (womb) that opens at the top of the vagina. Abnormal changes in the cells on the surface of the cervix is called cervical dysplasia.
How the Test is Performed
This procedure is done in the hospital or in a surgery center. During the procedure:
- You will be given general anesthesia (asleep and pain-free), or medicines to help you relax and feel sleepy.
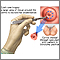
- You will lie on a table and place your feet in stirrups to position your pelvis for exam. The health care provider will place an instrument (speculum) into your vagina to better see your cervix.
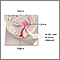
- A small cone-shaped sample of tissue is removed from your cervix. The procedure may be performed using a wire loop heated by electrical current (LEEP procedure), a scalpel (cold knife biopsy), or a laser beam.
- The cervical canal above the cone biopsy may also be scraped to remove cells for evaluation. This is called an endocervical curettage (ECC).
- The sample is examined under a microscope for signs of cancer. This biopsy may also be a treatment if the provider removes all of the diseased tissue.
Most of the time, you will be able to go home the same day as the procedure.
How to Prepare for the Test
You may be asked to not eat or drink for 6 to 8 hours before the test.
How the Test will Feel
After the procedure, you may have some cramping or discomfort for about a week. For about 4 to 6 weeks avoid:
- Douching (douching should never be done)
- Sexual intercourse
- Using tampons
For 2 to 3 weeks after the procedure, you may have discharge that is:
- Bloody
- Heavy
- Yellow-colored
Why the Test is Performed
Cone biopsy is done to detect cervical cancer or early changes that lead to cancer. A cone biopsy is done if a test called colposcopy cannot find the cause of an abnormal Pap smear.
Cone biopsy may also be used to treat:
- Moderate to severe types of abnormal cell changes (called CIN II or CIN III)
- Very early stage cervical cancer (stage 0 or IA1)
Normal Results
A normal result means there are no precancerous or cancerous cells in the cervix.
What Abnormal Results Mean
Most often, abnormal results mean that there are precancerous or cancerous cells in the cervix. These changes are called cervical intraepithelial neoplasia (CIN). The changes are divided into 3 groups:
- CIN I -- mild dysplasia
- CIN II -- moderate to marked dysplasia
- CIN III -- severe dysplasia to carcinoma in situ
Abnormal results may also be due to cervical cancer.
Risks
Risks of cone biopsy include:
- Bleeding
- Incompetent cervix (which may lead to premature delivery)
- Infection
- Scarring of the cervix (which may cause painful periods, premature delivery, and difficulty getting pregnant)
- Damage to the bladder or rectum
Cone biopsy may also make it hard for your provider to interpret abnormal Pap smear results in the future.
Alternative Names
Biopsy - cone; Cervical conization; CKC; Cervical intraepithelial neoplasia - cone biopsy; CIN - cone biopsy; Precancerous changes of the cervix - cone biopsy; Cervical cancer - cone biopsy; Squamous intraepithelial lesion - cone biopsy; LSIL - cone biopsy; HSIL - cone biopsy; Low-grade cone biopsy; High-grade cone biopsy; Carcinoma in situ-cone biopsy; CIS - cone biopsy; ASCUS - cone biopsy; Atypical glandular cells - cone biopsy; AGUS - cone biopsy; Atypical squamous cells - cone biopsy; Pap smear - cone biopsy; HPV - cone biopsy; Human papilloma virus - cone biopsy; Cervix - cone biopsy; Colposcopy - cone biopsy
References
Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169-182. PMID: 30638582 pubmed.ncbi.nlm.nih.gov/30638582/.
Salcedo MP, Phoolcharoen N, Schmeler KM. Intraepithelial neoplasia of the lower genital tract (cervix, vagina, vulva): etiology, screening, diagnosis, management. In: Gershenson DM, Lentz GM, Valea FA, Lobo RA, eds. Comprehensive Gynecology. 8th ed. Philadelphia, PA: Elsevier; 2022:chap 29.
Watson LA. Cervical conization. In: Fowler GC, ed. Pfenninger and Fowler's Procedures for Primary Care. 4th ed. Philadelphia, PA: Elsevier; 2020:chap 128.
Review Date 3/31/2024
Updated by: LaQuita Martinez, MD, Department of Obstetrics and Gynecology, Emory Johns Creek Hospital, Alpharetta, GA. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team.